Our natural understanding of matter is very self-centered. When ascribing properties to substances, we are quite subjective in our conclusions. We say rubber is elastic because it exhibits elasticity under the conditions we typically see. We think of ice as a solid because we are far more used to encountering it inside our freezers than on a flowing glacier. Furthermore, our perception of time biases us from seeing the motion of a glacier in the same light as the flowing of, say, honey.
It is natural for science to attempt to codify and explain these natural categorizations. Schoolchildren are treated to a relentless drumming of the basic “definition” of a solid as something that has a definite shape and does not flow. Similarly, students are told that liquids flow and have no definite shape but do have a definite volume while gases have neither definite shape nor volume.
Are Solids so Simple? Some Elementary Objections
On their own, these efforts at codifying the states of matter are reasonably accurate if you are speaking of practical experience, but science aspires to more precision and objectivity than this. Science attempts to describe how the world works in a non-self-absorbed way, and in this sense even these heuristics are rather lacking. In particular, solids do not have a definite shape. A piece of paper is a solid material, but you can fold it and it gets a new shape. In fact, I don’t even have to bend it; allow a sheet of paper to drape over the edge of the desk and it will have a different shape than it did when it was lying flat. As to whether solids “flow” or not, that depends on how you define the term. What makes the deformation of a stretched bungee cord any different than the deformation of honey poured from a bottle?
To address these examples, a science teacher who wants to toe the party line has to act much like the spokesman for a politician with a penchant for impolitic remarks. Each separate example gets its own excuse and explanation, but those explanations do not have a consistent message:
Reporter 1: Why did Mr. Textbook say that a solid has a definite shape when you can bend a piece of paper?
Spokesman: Mr. Textbook wasn’t referring to all types of forces acting on a solid; just gravity.
Reporter 2: What about the bungee cord?
Spokesman: What Mr. Textbook meant was that a solid doesn’t change shape based on its own weight.
Reporter 3: What about the paper draped over the edge?
Spokesman: Mr. Textbook wasn’t talking about temporary changes. He just meant that a solid will go back to its shape once the force is removed.
And at this point Reporter 1 and Reporter 3 stare at each other in disbelief.
A More Accurate Mantra, and More Significant Issues
The objections raised in the previous section are, in some sense, elementary. They are mostly semantic in nature, but I believe they are still relevant. If we are going to have students accept a mantra (“A solid is …”) and repeat it over and over every grade, we should at least get the mantra right or be clear what we mean.
A more accurate way of conveying what textbooks mean would be to say something like “Solids don’t drip.” It avoids the red herring of “definite shape” and focuses on the notion of “flow” while combining with it the idea of non-elasticity. A bungee cord is critically different from honey in that a bungee cord, once it snaps, will return to its original shape. A drop of honey, after it falls, will remain a drop, not returning to whatever shape the honey had before it was poured. We say that liquids are viscous while solids are elastic because of this difference.
Using the clause “solids don’t drip” to differentiate between liquids and solids gets around the elementary problems with the textbook definitions of solid, liquid, and gas, but it cannot cope with a more severe problem. In essence, science education has painted itself into a corner by trying so hard to link states of matter with temperature.
Readers of Science Myths Unmasked Volume 2 will know that most of what is learned in school about temperature and phase change is wrong. Solids do not change to liquids at the “melting point.” Liquids do not boil at the “boiling point,” and temperature does not determine phase. The story told in textbooks is a fairy tale designed to help students remember certain facts at the expense of understanding what is going on.
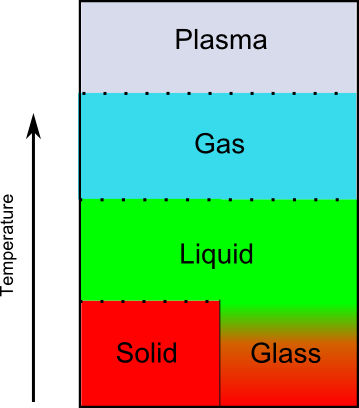
When it comes to teaching about states of matter, the interest in pinning so much on temperature actually distorts the entire picture given to students from the beginning. The textbook presentation is committed to a story where there are clear breaks between phases, occurring at set temperatures, and this puts kids on the wrong path from the beginning. The textbook regime only works for crystal solids, like iron, ice, and diamond. It doesn’t work for amorphous materials like wood, glass, and rubber. Since the textbook narrative only works for crystal solids, those are the examples students are shown. When discussing phases and phase changes, students are regularly provided illustrations of solids as comprising small molecules packed into well-defined lattices. The explanations of melting and other physical properties associated with solids tend to reinforce this incomplete picture.
A more accurate depiction of states of matter would make a clear distinction between crystal solids and amorphous materials that are designated as glasses by scientists. These are objects that seem solid but do not have a regular crystal structure. (Not all amorphous solids are glasses, but the exceptions are mostly materials that do not undergo typical phase changes. A simple example is wood. We don’t speak of wood “melting” because it undergoes chemical reactions well before the physical reaction of deliquescence. )
There are two reasons why the above picture would upend textbook orthodoxy, and both have to do with the outsize role given to temperature. First, this diagram suggests (quite rightly) that temperature does not determine phase. A substance can start out as a solid, be heated into a liquid, and then (upon cooling) become a glass, moving from the region marked “Solid” in the diagram, around the vertical, solid line, to wind up (at the same temperature) in the “Glass” region. If you take liquid water and cool it in one way, you get solid ice, like the cubes in your freezer. If you take that same liquid water and cool it in a different way, you get a different substance called glassy water. The transitions that substances make depend on more than temperature. (Note that there are several other reasons why temperature doesn’t determine phase. Those are not captured by this diagram but are discussed in Science Myths Unmasked Volume 2.)
The second reason this diagram would be heretical in standard science education is that textbooks want to hammer into students’ skulls the idea that changes in state occur at particular temperatures. In the above illustration, we see that the glass state transitions to the liquid state gradually. In fact, the “glass” state is not even considered a separate phase from the liquid in the normal sense of the word “phase.” (There are properties liquids have that objects in glass form do not have, but they are very different from the properties used to differentiate (crystal) solids from liquids.)
In many ways, “glasses” are better seen scientifically as very slow-flowing liquids than as solids, but we humans (being very self-absorbed in our labeling) tend to think of them more as solids than liquids because they react like solids in most day-to-day encounters.
An excellent demonstration showing how amorphous solids are like liquids is the “Pitch Drop Experiment,” which you can read about and see live here . This demonstration shows the fluid properties of pitch, a rubbery substance used to waterproof boats. It takes about 9 years for a drop to form. In our day-to-day experience, pitch acts like a solid, but on a longer timescale, it acts like a liquid.
Notably, this notion that glasses can be seen as simply “slow-flowing” liquids has more depth to it than you might think. A key notion separating liquids from solids is that solids often return to their original shape after being deformed while liquids do not. However, the response of a material to a deformation heavily depends on how rapidly the deformation occurs. A material can act like a solid at 20 degrees C when it is deformed at a certain rate but act like a liquid at the same temperature when deformed at a different rate.
This principle, called Time-Temperature Superposition, has real application in materials science. A scientist can measure the elastic properties at one temperature at a particular rate of deformation and predict how the same material will act at a different temperature at a different rate of deformation.
Of course, I cannot help but point out that this gives yet another example of how temperature does not, by itself, determine changes in the properties of matter.
(Note: A careful reader might wonder about the flowing of ice in glaciers given that ice is a crystal substance. The dynamics causing ice to flow in a glacier are fundamentally different from the flow seen in the pitch-drop experiment. It is not clear whether a small sample of ice would ever flow in this way if it were kept in conditions preventing liquefaction. Still, the example is good to bear in mind when considering the absolute statements made in textbooks, and we could make a claim that describing a liquid as something that “flows” is itself a fundamental error.)